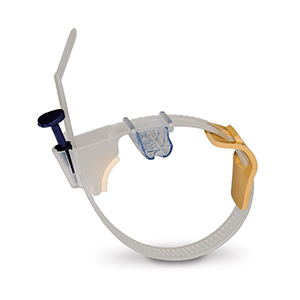
Radial Hemostasis
RadAR™ Vascular Compression Device
Product Overview
The sterile, single-use RadAR™ Devices provide economical, safe hemostasis for your post-catheterization radial access patients. In all RadAR models, the user can quickly adjust compression to permit patent blood flow through the artery and gradually release pressure during hemostasis, without unfastening the device. This enables patent hemostasis which has been shown to reduce the incidence of chronic radial artery occlusion. (1)
US Pat. No. 9,924,949
(1) Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of Radial Artery Occlusion-Patent Hemostasis Evaluation Trial (PROPHET Study): A Randomized Comparison of Traditional Versus Patency Documented Hemostasis After Transradial Catheterization. Catheterization and Cardiovascular Interventions 72:335-340 (2008)
The RadAR™ Vascular Compression Device is indicated for use by medical professionals to promote hemostasis following a catheterization or other puncture into a blood vessel in a patient’s arm or leg, including: radial, brachial, dorsalis pedis or tibial blood vessels, arterial or venous line or sheath removal, hemodialysis, and in patients on anticoagulation therapy.
| Item | Description | MOQ | Qty |
|---|---|---|---|
| 140-0150-00 | Model 4150, RadAR Vascular Compression Device, Sailor Blue | Box | 25/each/box |
| 140-0160-00 | Model 4160, RadAR Vascular Compression Device, Natural | Box | 25/each/box |